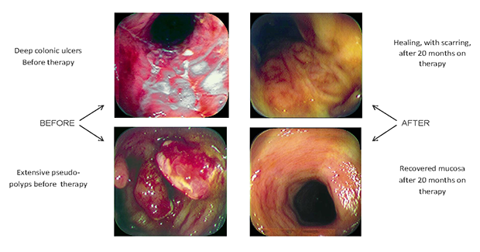
This is good news for people looking for an alternative therapy option for hard to treat cases of ulcerative colitis (UC). Read the case about the man with UC under the Clinical Highlight section of this article. It's hopeful in my opinion.
My $0.02 - It's nice to see herbal forms of medicine being tested clinically and seeing the results published in known journals. I'd like to see a larger study though. The 1st thing a skeptic would point out about this research is the size of the study. It's way too small, but not small enough to say that the results don't show significance. There just needs to be larger clinical studies being conducted to make an impact. The more participants that have a beneficial result, the more noteworthy the findings will be. Size matters in this case, especially since TCM and naturopathic medicine are not recognized as effective in the medical community........ still. You would think that by now alternative approaches of treating diseases would be accepted more than they are. Unfortunately the pharmaceutical presence is all we can see and hear because the industry's ability to market their drugs.
When we are faced with a choice in respects to treatment (alternative approach vs. conventional), we have to take into consideration the two types of areas of medicine and the facts surrounding them. This part is simple. Large markets get more exposure. More exposure leads to something being talked about more, used more, and becoming the popular choice that is opted for the masses. Who has never heard of Cymbalta, Humira, Lunesta? If you have a tv, you've heard of at least one of these drugs. Are the drugs that we hear about on a constant basis a better choice than the little guy that doesn't have the multimillion dollar advertising budget? You make that decision. Research and figure it out.
New research finds a very powerful Traditional Chinese Medicine (TCM) herb effective in resolving ulcerative colitis. The patients examined in the study published in the World Journal of Gastroenterology suffered from intractable ulcerative colitis and were unresponsive to conventional drug therapy. After use of the herbal medicine, 6 of the 7 patients in the study were able to completely discontinue the use of anti-inflammatory medications. This included the use of aminosalicylates, corticosteroids and azathioprine. Endoscopy and symptomatic responses showed everything from significant clinical improvements to a complete resolution of the condition.
Herbal Powder
The patients orally self-administered 1 gram of Qing Dai (Indigo Naturalis) powder, 2 times per day for 4 months. The results of the herbal program demonstrated significant clinical and objective improvements such that 6 of the 7 patients completely discontinued the use of prednisolone, a corticosteroid used to control ulcerative colitis. The researchers examined Qing Dai to learn more about its effective mechanisms of action. Using electron spin resonance, they discovered that Qing Dai has potent hydroxl radical scavenging activity. This discovery prompted the researchers to recommend further investigation into the mechanisms of Qing Dai’s anti-inflammatory effects.
The patients orally self-administered 1 gram of Qing Dai (Indigo Naturalis) powder, 2 times per day for 4 months. The results of the herbal program demonstrated significant clinical and objective improvements such that 6 of the 7 patients completely discontinued the use of prednisolone, a corticosteroid used to control ulcerative colitis. The researchers examined Qing Dai to learn more about its effective mechanisms of action. Using electron spin resonance, they discovered that Qing Dai has potent hydroxl radical scavenging activity. This discovery prompted the researchers to recommend further investigation into the mechanisms of Qing Dai’s anti-inflammatory effects.
This research coincides with other recent research demonstrating that acupuncture and herbal medicine are effective in the clearing of chronic ulcerative colitis. Published in the Clinical Journal of Chinese Medicine, the study showed that a combination of herbal enemas consisting of Ku Shen and Bai Tou Weng combined with an acupuncture treatment regime was significantly more effective than taking antibiotics for resolving ulcerative colitis.
The Qing Dai study examined the oral administration of Qing Dai in its powdered form. Qing Dai has received a great deal of attention in modern research. One recent research study found that I3M, synthesized from the indirubin found in Qing Dai, downregulates cancerous tissues when applied topically to oral cancer. This shows great potential for the treatment of oral cancer. Historically, TCM documents Qing Dai as an important herb in the treatment of ulcers in the mouth and tongue. TCM also documents the use of Qing Dai as a topical paste for the treatment of acne and topical ointment for the treatment psoriasis. HealthCMi recently published instructions on how to prepare the anti-acne topical paste in its blog section. Visit the Healthcare Medicine Institute's blog to learn more.
The I3M study cited the TCM formula Dang Gui Long Hui Wan as an historically important herbal compound for the treatment of chronic myelocytic leukemia. Many sources include Qing Dai as one of the ingredients in this formula that contains Dang Gui, Long Dan Cao, Zhi Zi, Huang Lian, Huang Bai, Huang Qin, Lu Hui, Da Huang, Qing Dai, Mu Xiang, She Xiang and Sheng Jiang. The researchers suggest that the indigo dye found in Qing Dai is partially responsible for the herbal formula’s efficaciousness given the modern research demonstrating that indirubin powerfully inhibits several types of human cancer cells. The Qing Dai researchers noted that modern studies demonstrate that indirubin has anti-inflammatory effects by suppressing interferon-alpha, interleukin-6 and nuclear factor. They added that Qing Dai has been shown to exert anti-inflammatory “effects on human neutrophils based on its ability to suppress superoxide generation.”
Clinical Highlight
The Qing Dai study highlighted specific clinical results of its participants. One patient vignette was of a man suffering from ulcerative colitis with hematochezia, the passage of fresh blood through the anus. This patient had taken antibiotics and prednisolone to control the hematochezia. However, he was unable to reduce the dosage of prednisolone without the return of hematochezia. Over time, the patient needed to increase the drug dosages to maintain clinical results and he showed no clinical improvements in his baseline condition. After 3 years, he began the Qing Dai treatments and after one month the hematochezia resolved completely. Objective testing also showed a marked decrease in serum C-reactive protein levels. The patient was able to discontinue the use of all drugs. Endoscopy revealed that his ulcers completely disappeared. A follow-up confirmed that the therapeutic effect of Qing Dai therapy lasted for more than 2 years.
The Qing Dai study highlighted specific clinical results of its participants. One patient vignette was of a man suffering from ulcerative colitis with hematochezia, the passage of fresh blood through the anus. This patient had taken antibiotics and prednisolone to control the hematochezia. However, he was unable to reduce the dosage of prednisolone without the return of hematochezia. Over time, the patient needed to increase the drug dosages to maintain clinical results and he showed no clinical improvements in his baseline condition. After 3 years, he began the Qing Dai treatments and after one month the hematochezia resolved completely. Objective testing also showed a marked decrease in serum C-reactive protein levels. The patient was able to discontinue the use of all drugs. Endoscopy revealed that his ulcers completely disappeared. A follow-up confirmed that the therapeutic effect of Qing Dai therapy lasted for more than 2 years.
The researchers note that other related research finds important clinical results from the use of Qing Dai. Yuan, et al, discovered that Qing Dai enemas are clinically effective for the treatment of chronic hemorrhagic radiation proctitis. Given the recent research combining acupuncture with herbal medicine demonstrating that enemas of Ku Shen combined with Bai Tou Weng are effective for the treatment of ulcerative colitis, it may be consistent that adding Qing Dai to the enema will enhance its therapeutic effects.
Distinct from Qing Dai used as a one herb formula for the treatment of chronic ulcerative colitis is its use within herbal formulas within the scope of Traditional Chinese Medicine (TCM). Differential diagnostics within the TCM system recognise Qing Dai’s appropriate application for this biomedically defined disorder in cases of Heat in the Blood, Damp Heat and Heat and Toxins. However, some clinical presentations of chronic ulcerative colitis may be due to cases of cold and deficiency. In these instances, herbal medicines with very different biological functions may exert more effective clinical actions for the treatment of chronic ulcerative colitis.
How serious is this research? Below is an image of three colon wall sections that are inflamed due to chronic ulcerative colitis. The image speaks to the importance of integrating TCM herbal medicine into the healthcare system.
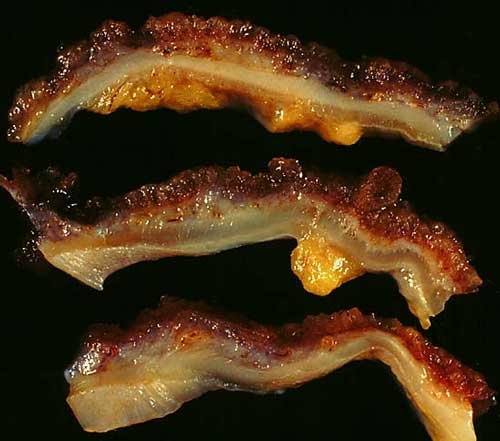 Colon wall inflamed from ulcerative colitis
Colon wall inflamed from ulcerative colitisReferences:
Suzuki, Hideo, Tsuyoshi Kaneko, Yuji Mizokami, Toshiaki Narasaka, Shinji Endo, Hirofumi Matsui, Akinori Yanaka, Aki Hirayama, and Ichinosuke Hyodo. "Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis." World journal of gastroenterology: WJG 19, no. 17 (2013): 2718.
Suzuki, Hideo, Tsuyoshi Kaneko, Yuji Mizokami, Toshiaki Narasaka, Shinji Endo, Hirofumi Matsui, Akinori Yanaka, Aki Hirayama, and Ichinosuke Hyodo. "Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis." World journal of gastroenterology: WJG 19, no. 17 (2013): 2718.
Clinical observation on treating chronic ulcerative colitis with retention enema by Baitouweng Kushen decoction and acupuncture, Clinical Journal of Chinese Medicine, 1674-7860, 2013.
Lo W-Y, Chang N-W (2013) An Indirubin Derivative, Indirubin-3′-Monoxime Suppresses Oral Cancer Tumorigenesis through the Downregulation of Survivin. PLoS ONE 8(8): e70198. doi:10.1371/journal.pone.0070198.Editor: A. R. M. Ruhul Amin, Winship Cancer Institute of Emory University, United States of America.
Yuan G, Ke Q, Su X, Yang J, Xu X. Qing Dai, A traditional Chinese medicine for the treatment of chronic hemorrhagic radiation proctitis. Zhong De Linchuang Zhongliuxue Zazhi. 2009;8:114–116.
Lin YK, Leu YL, Huang TH, Wu YH, Chung PJ, Su Pang JH, Hwang TL. Anti-inflammatory effects of the extract of indigo naturalis in human neutrophils. J Ethnopharmacol. 2009;125:51–58.